Observation Services
MOOSE
DYFAMED station in the Ligurian Sea
The DYFAMED-BENTHOS survey was established in December 1990 at the DYFAMED permanent station in the Ligurian Sea (NW Mediterranean) to investigate benthic to pelagic coupling, and particularly benthic biological (with special emphasis on metazoan meiofauna) and biogeochemical responses to varying particulate organic matter inputs to the sea bed. For a detailed description, see Guidi-Guilvard (2002).
The DYFAMED permanent station (43°24’N -7°52’ E) in the northwestern Mediterranean (>2300 m) is relatively close to land (28 nautical miles off Nice) but presents many of the characteristics of the open ocean with a strong surface seasonal signal and essentially vertical particle fluxes. It is the study site of the on-going French MOOSE (formerly DYFAMED) Observation Service, providing comprehensive background water column data.
The benthic site lies on the western side of the lower middle valley of the Var Canyon deep-sea fan. The terrain is relatively flat (2347 ± 6 m depth) with muddy surface sediments (silt-clay fraction >94% of sediment dry weight) derived primarily from pelagic sedimentation. The bottom-water temperature is high (12.7°C), salinity is elevated (38.4‰) and average dissolved oxygen
concentration is 4.7 ml l-1 (Guidi-Guilvard, 2002). During the benthic survey (1990 - 2003) that comprised different
phases based on sediment sampling resolution (see Guidi-Guilvard, 2002), sediment samples were collected monthly to bimonthly for
5 consecutive years (1993 - 1997). Here, are shown meiofauna data from preliminary investigations (December 1990 - December 1992)
as well as from the first 3 years of the "high frequency survey" (1993 - 1995) during which the average sampling frequency was 40 days
(Guidi-Guilvard & Dallot, 2014).
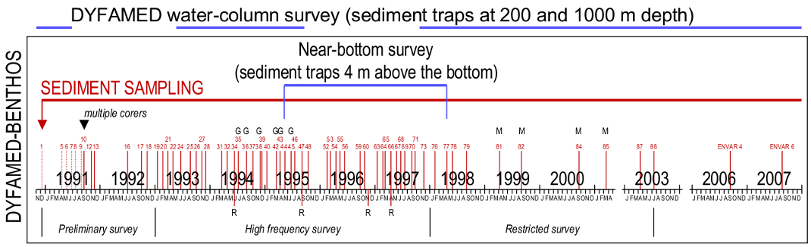
Diagram of the DYFAMED-BENTHOS (DYFB) sampling design. Vertical bars represent the 64 benthic cruises carried out for 13 years, from late 1990 through 2003, and during which surface sediment was successfully sampled at a mean depth of 2347 m, using either a box corer (dashed bars) or a multicorer (solid bars). The program was split into 3 phases, depending on sediment-sampling frequency. Cruises including collaborations with geochemists (G) and microbiologists (M) are indicated. A free-vehicle benthic respirometer (R) was deployed on 4 occasions between 1994 and 1997, while sediment traps collected particles in close proximity to the bottom between February 1995 and April 1998 to supplement the information obtained from the upper water column DYFAMED survey that was moreover interrupted twice for a 2-year period. The station was furthermore sampled in 2006 and 2007 during two additionnal cruises (ENVAR 4 and 6).
Surface sediment was first collected using a box corer. From September 1991 on, it was sampled with a Barnett-Watson-type multiple corer (Barnett et al., 1984) and a Bowers and Connelly Maxicorer (see Eleftheriou, 2013). Both devices yield virtually undisturbed sediment samples. They were equipped with a set of eight 60-cm-long core tubes with an inner diameter of 9.5 and 9.8-cm, respectively. They had an inter-core separation ranging from 17 to 68 cm (distances are from the centre of the tubes). Shipboard positioning was carried out using DGPS (Differential Global Positioning System), which can yield horizontal accuracies of 1 to 10 m. To minimise spatial variability, the bottom target aimed at for coring was a ~200-m diameter circle centred on 43°24.62’ N - 7°51.68’ E in which over 80% of the multicorer deployments were achieved. On each cruise, from 1 to 3 corer casts were achieved. Approximate distance from one multicorer to another ranged from 10 m to 335 m.
Meiofauna sample analyses
Metazoan meiofauna was subsampled using 60-ml cut-off syringes (6-cm2 cross sectional area) to a sediment depth of 10 cm, with 2 cm of overlying water. One to 3 subsamples were collected from 1 to 4 core tubes from each deployment. The inter-subsample separation was roughly 3.5 cm (from the centre of the syringes). Samples were stored in filtered seawater at 2°C for a few hours to relax animals before they were preserved by adding borax-buffered formalin to a final concentration of 4%. In the laboratory, they were stained with Rose Bengal, stored in the dark, and later gently washed with filtered tap water through a 40-µm sieve. The organisms retained on the sieve were extracted from the remaining sediment by density gradient separation using Ludox TM-40 (in McIntyre and Warwick, 1984). The specific gravity of Ludox was increased to 1.20 in order to extract even the heaviest organisms such as juvenile bivalves. After a thorough wash on the sieve, the residue was examined under an Olympus SZH stereo microscope (96 x magnification) and the metazoans were counted and identified to the major taxon level (Higgins and Thiel, 1988).
Eleftheriou, A., (Ed.), 2013. Methods for the study of marine benthos, fourth edition. Wiley Blackwell, Chichester.
Guidi-Guilvard, L.D., 2002. DYFAMED-BENTHOS, a long time-series benthic survey at 2347 m depth in the NW Mediterranean: general introduction. Deep-Sea Research II 49, 2183-2193.
Guidi-Guilvard L.D., Dallot, S., 2014. Metazoan meiobenthos temporal fluctuations in the deep NW Mediterranean Sea (DYFAMED-BENTHOS 1993-1995). Contribution of spatial variability and disturbance. Deep-Sea Research I, 92: 127-140.
Higgins, R.P., Thiel, H. (Eds.), 1988. Introduction to the study of meiofauna. The Smithsonian Institution Press, Washington D.C., pp. 61-78.
McIntyre, A.D., Warwick, R.M., 1984. Meiofauna techniques. In: Holme, N.A., McIntyre, A.D. (Eds), Methods for the study of marine benthos, Blackwell Scientific Publications, London, pp. 217-244.








